Silver powder produced by Kunshan Yosoar
The silver powder produced by Kunshan Yosoar is prepared by evaporation and condensation. The silver powder produced has a relatively high sphericity and a high purity. The particle size can also meet your customized needs, and the distribution is relatively uniform.
Silver powder has several shapes, such as flake, dendritic, and spherical.
The SSA of flake silver powder is larger than that of spherical silver powder.
The surface activation of flakes is lower than that of spherical silver powder.
The smaller the particle size of the silver powder, the stronger the bactericidal performance.
The surface of the conductive layer of the flake silver powder is flat and has good conductivity.
Nano silver particles have good surface effect, excellent conductivity, and quantum size effect.
Used in electronic paste
Silver powder can be used in the electronic paste industry. Among them, there are many ultrafine silver powders, which can be used in electronic pastes. Generally, spherical and flake silver powders are selected.
Silver powder can be made into a paste. Generally, the size of the particle size will affect the characteristics and conductivity of the silver paste. When using silver powder, the particle size should be controlled within an appropriate range.


Used in antibacterial products
Nano silver powder can be used in antibacterial products because it has a large specific surface area and small-scale effect. Compared with general inorganic antibacterial agents, it has better antibacterial effect, high safety, and durability.
Silver powder is very active, has strong antibacterial ability, and can kill many bacteria, fungi and other pathogenic microorganisms.
Yosoar: conductive silver paste Supplier in China
Kunshan Yosoar’s conductive silver powder is currently mainly used to make conductive silver paste, or made into nano-silver dispersion, which can play a good bactericidal effect.
The silver powder produced by Kunshan Yosoar, because of its good electrical conductivity and antistatic effect, can replace copper powder in some application fields and become the main product. Silver powder can be used for conductive films, conductive fibers, and can also be mixed into conductive adhesives. .
In addition, the silver powder is also utilized in polymer plastic substrates. The conductive paste made of silver powder can also be used as a catalyst or antibacterial material after sintering. Apart from this, it can also be used for general conductive coatings and inks, as well as electrical conductivity and electromagnetic shielding related to electronic products.
- Manufacturing
- Certification
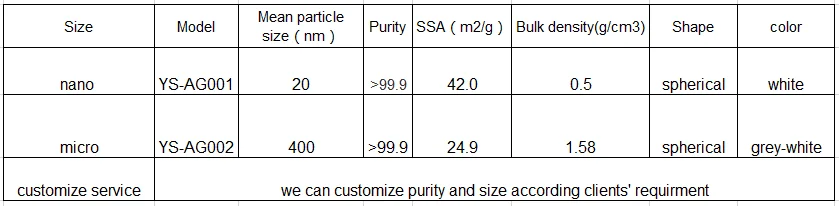
Model of silver powder
This method of preparing high purity silver powder and also can be prepared by nano-silver powder, suitable for mass production. However, the equipment requirements are high, the equipment structure is complicated, and if we consider the overall cost thing, then it is relatively high.
Spray pyrolysis (Spray Pyrolysis, abbreviated SP method ) is known as solvent evaporation decomposition. In this method, the silver-containing solution is sprayed into a high-temperature atmosphere ( which can be sprayed into a heated reactor or a high-temperature flame ). The high temperature causes the solvent to evaporate and thermally decompose the silver salt to produce a Ag powder. This method has a strong production capacity, simple technological process, continuous operation and fast production efficiency. If we consider the size of the particles of this Ag powder, then it is also relatively wide. It has indeed got a wide particle size distribution.
The liquid phase reduction method to prepare silver nanopowder( Ag powder) basically refers to the general process of reducing and depositing silver particles from silver salt, silver complex aqueous solution, or organic system with a reducing agent in the liquid phase. In essence, it is a comprehensive process of kinetics, thermodynamics, electrochemistry, and fluid mechanics. This process also determines the overall physical as well as the chemical characteristics of the powder, such as particle size, crystallinity, chemical purity, and agglomeration status.
The process flow is simple, the requirements for reaction equipment are not high, and the reducing agent, dispersant, etc. can be flexibly selected, which is suitable for small-scale experiments and large-scale production. The surface finish of the prepared Ag powder is not high, and the solid-liquid separation is difficult under certain conditions.
Under normal circumstances, the silver salts used are: silver nitrate (AgN03), silver ammonia complexion and silver potassium cyanide (KAg(CN)2), etc. The choice of reducing agent: hydrated off, formaldehyde, ascorbic acid, glucose, sodium hypophosphite, glycerol, boots acids, organic amines, shuttle acid, triethanolamine, unsaturated alcohols, and the like, commonly used dispersants and protective agents are: ten Sodium dialkyl sulfate ((SDS), gelatin, oleic acid, polyvinylpyrrolidone (PVP), sorbitol, polyvinyl alcohol ((PVA)), triethanolamine, etc. Through the precursor, reducing agent, preparation process With the adjustment of equipment, it is possible to prepare metallic silver powder with a size ranging from nanometer to micrometer and spherical or polyhedral morphology.
Improve cleanliness and productivity; 2. Controllable morphology, the overall particle size as well as the general distribution of particle size; 3. Make separation and collection easy to operate.
On its growth mechanism, the industry has reached a rough consensus. It is generally believed that the growth path of silver particles can be divided into the following stages: Reaction: 1 Silver ions are reduced to produce silver atoms; 2 Nucleation: Ag atoms aggregate to form nuclei; Silver nuclei Accumulate to form seed crystals ; 3 growth: the atoms of silver are gradually deposited on the overall surface of the crystals of seed and this is done in order to promote the growth of the seed crystals and become crystals; 4 agings: nanocrystals aggregate and grow to become self-assembled particles.
At present, the situation in the preparation process of silver paste is roughly the same, and the characteristics of nano-silver paste are not only the same as compared to those of nano-silver paste.
The size, shape and filling material of the particles are related, and the stability of the final performance of the silver nanopowder paste is also the same as compared to that of the organic dispersion medium,
Dispersant, pH value, shear rate, shear time, etc. also have a great relationship; the agglomeration phenomenon in nano-silver paste affects
A very important factor affecting the stability of nano-silver paste, preventing the precipitation and agglomeration of the paste, and improving the stability of the paste
It is the first condition for preparing nano-silver dispersion paste with good dispersibility.
The preparation of powder by emulsion has a good theoretical basis, but despite this fact, it is a bit difficult in order to obtain a stable emulsion during the actual operation of emulsion dispersion, which leads to the uncontrollable changes in the overall size as well as the morphology of the prepared silver powder to a certain extent, and a large amount of it will be added during the experiment. Organic matter is difficult to clean and centrifuge. This process will also affect the agglomeration of silver powder particles, so further exploration is needed.
During the liquid phase reaction, the nucleation as well as the growth of crystals are also accompanied by collisions and merging between particles. Due to the small grain size, a generally large specific surface area and also a comparatively huge quantity of unsaturated bonds and dangling bonds on the surface, the surface free energy is very high. The solid fine particles cannot freely change their surface shape and reduce the free energy like liquids, so they can only reduce the specific surface area by mutual anvil with the surrounding fine particles, to achieve a more stable state during the agglomeration. The main cause of agglomeration in this stage is the van der Waals force, electrostatic attraction and Brownian motion between particles, mainly soft agglomeration.
Since there is a large amount of liquid medium between the solid particles and the particles can repel, the agglomeration process is often reversible. The following measures can be taken to prevent and eliminate :
①Using electrostatic repulsion. The surface of the same kind of solid particles in the same liquid phase system will often carry the same kind of electric charge, causing electrostatic repulsion between each other. The electrostatic repulsion between particles can effectively inhibit the agglomeration between particles. The overall size and shape of the electrostatic repulsion between particles is determined by the thickness of the diffusion layer on the particle surface and the Zeta potential. Therefore, the precipitation process can be performed in a low ionic strength environment or a region far away from the isoelectric point by adjusting the temperature, pH value, and electrolyte concentration, to enhance the electrostatic repulsion between the particles to prevent agglomeration from occurring.
②Using the steric hindrance of the gel. The controlled precipitation reaction takes place in the gel, and the nucleation and growth of the product will occur in the gel; at this time, the steric hindrance of the gel will inhibit the collision and merging between the particles, thereby preventing agglomeration from occurring.
③Using the protective effect of dispersant. Dispersants include surfactants, hydrophilic polymers and compounding agents, which can form a protective layer after being adsorbed on the overall particles surface, thereby preventing the collision and merging between the particles. The use of dispersants to prevent agglomeration is the most common and effective method. Currently commonly used dispersants are polyvinyl alcohol ((PVA), polyvinylpyrrolidine copper ((PVP)), sodium dodecyl sulfate (SDS) and cellulose derivatives, etc. The electrostatic effect and steric effect are dispersants The main reason to prevent agglomeration between particles.
④Using the dispersion effect of external mechanical energy. Dispersion techniques such as stirring and ultrasonic vibration can also prevent and eliminate particle agglomeration in the liquid phase precipitation process.
The primary problem in the preparation of Ag powder is the depolymerization of the powder. Micro- and nano-Ag powders are in an unbalanced thermodynamic state as a result of their relatively small size of particles as well as high surface energy. The silver particles are prone to agglomerate to form secondary particles with larger particle sizes, that is, agglomerates. An effective solution is to add a dispersant during the preparation process. The selective adsorption characteristics and steric hindrance of the dispersant are also involved in playing an important role in the overall particle size and the degree of agglomeration between particles.
Compared with ordinary Ag powder, silver nanopowder has a size between atomic clusters and macroscopic particles, so it has the surface effect of nano-materials, volume effect, quantum size effect, macro-quantum tunneling effect and other special features that many macro-materials do not have. Nature.
The surface effect of nano-Ag powder also refers to the effect that is caused because of the general increase in surface area and it also effects the total number of surface atoms after silver becomes ultrafine powder. The ratio of surface atom number to total atom number of nano silver powder increases sharply as the overall size of particle decreases. Also, the surface area of this nano Ag powder can generally reach 1 ~ 10 m 2 / g. The change of specific surface area leads to a series of changes in properties, such as the melting point decreases as the particles become smaller, the thermal resistance of nano-Ag powder tends to zero at low temperatures, and the mechanical properties such as strength increase as the particles decrease. Also, the geometric configuration of the atoms on the surface of the nano-silver powder, the interaction between the atoms and the electronic energy spectrum are different from the inside, and they have great chemical activity. Therefore, the surface-related adsorption, catalysis, diffusion and sintering characteristics are different than compared to those of silver. Large particles are different.
The volume effect of nano silver powder basically refers to the general effect that is caused by the reduction in the volume and the reduction of the total number of particles atoms. As the total number of atoms in the silver powder decrease, their will be an apparent increase in the energy level interval of the energy band, and also some electrical, magnetic, thermal and other properties or characteristics will be abnormal. People can also intuitively perceive that the nano-Ag powder is black rather than being silver-white or generally being large-grained silver, and also, the smaller the particle size means that it will have more darker color. This is due to the proton vibration and discontinuity of energy levels appearing with the reduction of silver particles, and major changes in light absorption, emission and scattering.
As the particles size decreases, under low-temperature conditions, the nano-silver powder can exhibit a quantum size effect. Starting from the energy band theory, the energy spectrum of bulk metal conduction electrons is quasi-continuous. However, when the particle size decreases, the continuous energy bands will separate into discontinuous energy levels. When the distance between discrete energy levels is greater than thermal energy, magnetic energy, electrostatic energy, photon energy, and the condensing energy of superconducting states, an effect different from a macroscopic object will occur, which is called the quantum size effect. The silver nanoparticles in the presence of low temperature ” Cooper effect ” (KuboEffect), i.e. the energy level discontinuity properties. Particle size < 10 nm electrons ultrafine particles of about 10 . 4 th, between the reference level and the Fermi level energy states of about 1K, and therefore the Fermi level of energy equivalent to about 10 . 4 K. This means that due to the discontinuity of energy levels, the magnetic susceptibility, electrical conductivity, specific heat and nuclear magnetic relaxation of silver nanoparticles at low temperature ( liquid helium temperature ) are abnormal. At present, the quantum size effect has been confirmed by experiments such as magnetic measurement, nuclear magnetic resonance, electron spin resonance, and spectral line shift.
Electrons have both particle and volatility, and the ability to pass through a potential barrier is called the tunneling effect. Recently, it has been observed and discovered that some macroscopic physical quantities, such as the magnetization of nanoparticles, also have a tunneling effect. They can pass through the macroscopic barrier to produce changes. This is called the macroscopic quantum tunneling effect of nanoparticles.
Compared with silver powders of other morphologies, flake Ag powders are also utilized as conductive fillers when forming conductive paths, because the particles are in line or surface contact. Compared with the point contacts of spherical Ag powders, flake silver powders have relatively lower electrical resistance; at the same time; The specific surface area of flake silver powder is larger than that of spherical Ag powder, and its surface activation energy is much lower comparatively than that of spherical or spheroidal silver powder, so its degree of oxidation and oxidation tendency is lower; also, flake Ag powder has a wider range of deflection and resistance to flexural cracking and stretching Features can greatly improve the reliability of electronic components. Due to the above advantages of flake structure silver powder, it has excellent performance when preparing high-conductivity and low-temperature silver paste coatings, and it has the white luster of silver, so because of these characteristics, it has wide applications in various electronic components, such as carbon film potentiometer tips, Membrane switches, filters, solar electrodes, etc. Flake Ag powder can not only be used as a conductive functional material but also has a good application prospect in optics, catalysis and biomarking because of its remarkable size effect and shape-sensitive surface plasmon band.
What is the general antibacterial phenomenon of nano Ag powder?
The reason why nano-silver has received widespread attention is inseparable from its antibacterial activity. In recent years, many researchers have used electron microscopy, proteomics analysis, and recombinant bioluminescent bacteria to conduct a series of studies on the sterilization principle of nano-silver in terms of morphology, structure and physiological changes, protein level and transcription level. Provides a clear picture of the antibacterial phenomenon of silver powder. Studies believe that the general antibacterial effect of this nano-silver is a complex mechanism that simultaneously acts on the inside and outside of bacterial cells. Take E. coli as an example. When E. coli is exposed to nano silver, it can be seen through an electron microscope that generally most of these particles of nano silver( silver powder) are attached to the surface of the cell membrane of E. coli. Nano-silver damages and disturbs the overall physical structure and function of the cell membrane by interacting with the outer membrane barrier components (such as lipopolysaccharide and porin, etc.), changing the membrane potential and membrane permeability, causing the cell’s ion transport system to be disturbed; nano-silver It can enter the cell, causing the loss of potassium and phosphate in the cell, the hydrolysis of ATP in the cell, and the rapid decrease of the level. Also, nanoparticles release silver ions in an aqueous environment, which constitutes another source of toxicity to E. coli. Silver ions entering cells will induce the production of superoxide free radicals and other reactive oxygen free radicals. These free radicals can cause cell oxidative stress and cell membrane damage. Nano silver particles entering cells can also cause cell apoptosis or necrosis. Cell death. Nano-silver and Ag + cause changes in cell osmotic pressure, oxygen pressure, and pH, which in turn cause reactions such as protein denaturation in cells, and may cause DNA damage. The antibacterial activity of nano-silver is a synergistic effect of protein/membrane damage and oxidative damage, and this effect is related to the particle size. In contrast, micro-scale silver particles do not affect such as membrane damage. The observed bactericidal effect of silver ions is also achieved by causing the disturbance and rupture of the cell membrane, causing a large loss of protons and the collapse of membrane potential.
The antibacterial properties of metallic silver have been known since ancient times: the ancient Egyptians knew that thin silver sheets were used to cover wounds to prevent bacterial infections and accelerate wound healing; the Mongolians knew that silverware could keep goat milk fresh. 1 Silver-loaded activated carbon fiber can also be used for the purification and the disinfection of drinking water. It not only exerts the excellent adsorption performance of activated carbon fiber on organic matter but also increases the antibacterial and sterilization function of carbon fiber, which promotes the wide application of activated carbon fiber in drinking water purification. Japanese everyday life in a variety of plastic products, we have contacted has replaced metal products, plastic products, but very easy to breed bacteria and infection, so most likely caused by the spread of germs and cross-infection, so that the antibacterial plastic products processed into necessary. In the manufacturing process of plastic products, silver series antibacterial agents are added to the available plastic or fixed on the surface of the plastic to make the surface of the product have antibacterial and antifungal effects, and produce plastic products with antibacterial and antifungal surfaces. To save and effectively use antibacterial agents and reduce production costs, nano-silver antibacterial agents have received more and more attention from manufacturers. At present, most antibacterial agents are selected nano-Ag powders. Japanese everyday life more and more products containing nano silver material into our lives. For example: washing and toiletries, cutlery containers, baby supplies, deodorant, humidifiers, air withered, refrigerators, washing machines, electric words, toys and paper equipment and so on.
(1) Antibacterial fiber and antibacterial dressing. Nano-silver has a broad-spectrum antibacterial effect, high sterilization or antibacterial effect, excellent washing resistance, good thermal stability and chemical stability. In medical protection, antibacterial fibers play a role in blocking bacteria and molds. The role of. Nowadays, nano-silver antibacterial fibers have been applied or will be used in many fields: ①Nano -silver antibacterial fibers are made into the burn and scald dressings and used in burns, burns, skin grafts, naval firearms and seawater immersion treatment; ②used in nano-silver incision stickers, Manufacturing and application of nano-silver wound stickers in trauma, skin, surgery and incision infections; ③ Textiles and fiber products containing nano-silver antibacterial materials are widely used in hospitals. For example masks, coats, shoe covers, etc. Antibacterial textiles can prevent cross-infection and the spread of bacteria.
(2) Antibacterial medical equipment. Antibacterial materials can reduce the chance of infection, and antibacterial appliances are becoming more and more popular among doctors. For example antibacterial scalpels, tweezers, catheters, stainless steel clips and other appliances. When testing its antibacterial effect, it was found that stainless steel with nano-silver material on its surface has excellent antibacterial properties.
As the content of nano-silver powder in the coating increases, the sterilization rate is significantly improved, indicating that the content of silver powder has a greater impact on the sterilization performance. This is because in the same environment, the higher the silver powder content, the greater the number of silver ions dissolved, and the greater the number of bacteria killed by the silver ions. When the content of nano-silver powder is low, the location of a considerable part of the bacteria is not easily affected.



























