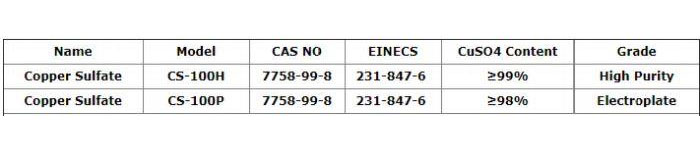
Copper Sulfate
Specific Gravity: 2.284
Solubility in Water: 22.37% at 0 °C (32 °F)
CAS Number: 7758-99-8…
Specific Gravity: 2.284
Solubility in Water: 22.37% at 0 °C (32 °F)
CAS Number: 7758-99-8…
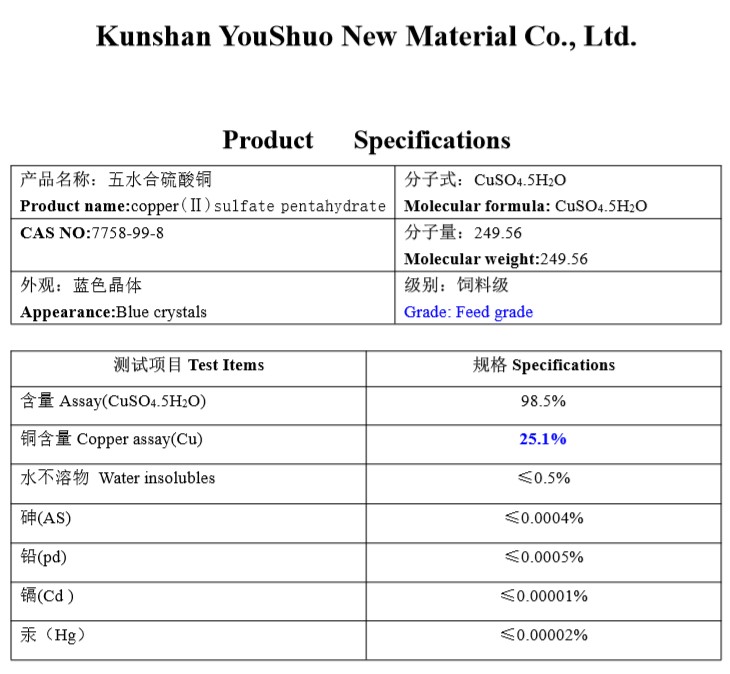
Chemical formula: CuSO4.5H2O
Molecular Weight: 249.69
CAS Number: 7758-99-8…
Boiling Point: 150 °C (302 °F)
Specific Gravity: 2.284
CAS Number: 7758-99-8…
Boiling Point: 150 °C (302 °F)
Specific Gravity: 2.284
CAS Number: 7758-99-8…
Specific Gravity: 2.284
Solubility in Water: 22.37% at 0 °C (32 °F)
CAS Number: 7758-99-8…
Specific Gravity: 2.284
Solubility in Water: 22.37% at 0 °C (32 °F)
CAS Number: 7758-99-8…
Boiling Point: 150 °C (302 °F)
Specific Gravity: 2.284
CAS Number: 7758-99-8…
Specific Gravity: 2.284
Solubility in Water: 22.37% at 0 °C (32 °F)
CAS Number: 7758-99-8…
Specific Gravity: 2.284
Solubility in Water: 22.37% at 0 °C (32 °F)
CAS Number: 7758-99-8…
Boiling Point: 150 °C (302 °F)
Specific Gravity: 2.284
CAS Number: 7758-99-8…
Boiling Point: 150 °C (302 °F)
Specific Gravity: 2.284
CAS Number: 7758-99-8…
Kunshan Yosoar New Materials Co.,Ltd
CuSO4.5H2O FAQ Guide:
What are the physical properties of copper sulfate?
The appearance of this is transparent dark blue crystal or powder. It is soluble in water but insoluble in ethanol and almost insoluble in most other organic solvents. It could be slowly weathered in the air when the environment is moist. The color of CuSO4.5H2O always yellow or green when it contains many impurities and has no smell. The density of anhydrous it is 3.603 g / cm3. It has strong water absorption and corrosive to iron.
What are the chemical properties of copper sulfate?
Displacement reaction-The metal whose activity is before copper can replace copper from CuSO4.5H2O solution. Thermal decomposition-copper sulfate easily absorbs water vapor in the air and becomes hydrate, and it loses crystal water after heating. When the heating temperature reaches 653 ℃, CuO and SO3 begin to decompose. Substitution reaction-When it reacts with ammonia water, a small amount of copper hydroxide is formed, and when it is excessive, the complex CuSO4.5H2O tetramine is formed.
What are the application fields of copper sulfate?
As an important inorganic raw material, it is widely used in agriculture, feed, water treatment, electroplating, catalyst, paint, mineral processing, and other industries.
It is most commonly used in agriculture. The mixture of CuSO4.5H2O and lime milk is a good fungicide, which can also be applied to control many kinds of crop diseases. Besides, the Cu ions in CuSO4 penetrate through the cell wall to replace the Mg ions in chlorophyll, which makes the chloroplast unable to carry out photosynthesis and then used for algae removal.

What matters needing attention when using CuSO4.5H2O?
Be careful not to eat CuSO4.5H2O by mistake and remember not to let copper sulfate get in touch with sensitive parts such as the eyes. After contact with sensitive parts, flush the area and rush to the hospital immediately.
Is CuSO4.5H2O harmful to the human body?
It is harmful to human health. When people eat CuSO4.5H2O by mistake, It can stimulate the gastrointestinal tract, and cause nausea, vomiting, copper taste in the mouth, and stomach burning sensation. Severe cases include abdominal colic, hematemesis, and black stool. It can cause serious renal damage and hemolysis, jaundice, anemia, hepatomegaly, hemoglobinuria, acute renal failure, and uremia. Irritating to eyes and skin. Long-term contact can cause contact dermatitis, irritation of nasal and ocular mucosa, and gastrointestinal symptoms.
What are the production methods of copper sulfate?
As a professional CuSO4.5H2O supplier, We have the following suggestion. First of all, the relevant operators need to receive professional training. In the specific operation process, they must strictly follow the regulations. Secondly, give workers as much physical protection as possible. Workers should wear dust masks, professional safety glasses, anti-poison work clothes, and rubber gloves. Others: smoking, eating, and drinking are not allowed at the work place. After work, each worker should shower and change clothes. Pay notice to personal hygiene. Carry out all the employment and regular physical examination.
What are the raw materials for making copper sulfate?
At present, the main production method of this is to produce CuSO4.5H2O from metal copper, which mainly includes copper powder, sponge copper, and scrap copper. Scrap copper mainly comes from copper scrap, abandoned copper wire, enameled wire, and other copper- containing materials. Scrap copper is actually a mixture of red copper, brass, and bronze. Its composition and content change with this, The composition of the three materials varies with the proportion. Copper ore is also the raw material when producing copper sulfate. It is mainly oxidized copper ore and sulfide copper ore.
Can copper sulfate be used in teaching?
Yes, it is not only used in crystal formation tests and copper electroplating tests but also often used to demonstrate exothermic reactions. Magnesium strip is inserted into CuSO4.5H2O solution during the demonstration. It can also be used to demonstrate the process of crystal weathering and crystal water production. In junior high school chemistry, the replacement reaction between copper sulfate and iron is used to verify the law of conservation of mass, and the physical change and chemical change are distinguished by crushing CuSO4.5H2O.

What are the precautions for transporting copper sulfate?
First of all, it is necessary to ensure the integrity of product packaging, pay attention to safety in the loading process, and it is forbidden to mix with other chemicals. In the process of transporting CuSO4.5H2O, it is necessary to ensure the tightness and stability of the container, so as not to cause leakage due to the damage of the container. The temperature of the container and the surface of the vehicle should be stable during transportation. After the transport is complete, the staff should thoroughly clean the vehicle to avoid the presence of residues.
What are the applications of copper sulfate in agriculture?
It is often used as an agricultural fungicide in agriculture. The Bordeaux mixture is made of CuSO4.5H2O aqueous solution and hydrated lime suspension. It is a kind of protective bactericide. The effective component is basic CuSO4.5H2O, which can effectively prevent spore germination, prevent pathogen infection, and promote the leaf color green, strong growth, improve the disease resistance of trees. The preparation has the characteristics of a broad bactericidal spectrum, long duration of efficacy, no resistance to pathogens, low toxicity to humans and livestock, etc. it is a kind of bactericide with the longest application history. At the same time, CuSO4.5H2O, as an inorganic compound, can be used as an effective drug in the treatment of copper deficiency. It can also be used as anthelmintic for cattle and sheep and emetic for pigs and dogs.

What are the applications of copper sulfate in the industry?
It can be used in the pigment industry to produce colorful dyes, such as reactive brilliant blue, reactive violet, etc. It also can be used as a catalyst for the synthesis of perfume and dye intermediates in the organic industry. In the chemical industry, It is also commonly used to make copper salts.
Sometimes, Copper oleate is employed as a harmful agent of protective paint for ship bottom within the coating trade. within the electroplating trade, it’s used as AN particle additive for sulphate copper plating and wide-temperature bright acid copper plating. Food grade is employed as AN antimicrobial and biological process supplement. it’s used as a chemical and copper chemical in agriculture.

What are the recommended chemical teaching supplies?
Our company is a chemical supplier of many universities, Based on our years of working experience, we recommend copper sulfate to you. It is widely used in teaching, its security is also reassuring. It can be used to demonstrate various physical and chemical experiments. Including but not limited to Exothermic reaction and Law of conservation of mass.
What are the characteristics of copper sulfate as fertilizer?
It is the most important copper fertilizer, Copper is one of the essential nutrients for plants, and it is absorbed by plants in the form of cation (Cu2 +). Copper can enhance photosynthesis, promote pollen germination and pollen tube elongation, and improve seed setting rate.

Which chemical can be used in artistic creation?
like a dark blue crystal, it is very beautiful and has a decorative effect, under the night light, the ornamental effect of copper sulfate crystal is better. Therefore, many artists prefer to use copper sulfate as the raw material of artistic creation.
What are the methods to deal with CuSO4.5H2O leakage?
The emergency personnel shall wear gas masks and gloves, isolate the contaminated area, and set up warning signs around. After washing with a large amount of water, the staff should discharge the diluted liquid waste into the wastewater system. If there is a large amount of leakage, the leaked CuSO4.5H2O should be recycled to the garbage disposal site for further treatment.
What storage precautions for copper sulfate?
It should be stored in a well-ventilated warehouse, and the staff should pay attention to keep the temperature of the warehouse stable. It also should be stored away from other chemicals to prevent chemical reactions after mixing.
What are the applications of CuSO4.5H2O in medicine?
It has the effects of emesis, deprivation, detoxification, treatment of epilepsy, and hemorrhoids in medicine, however, due to the toxicity is too large, there will be some side effects. It can also be used in medical experiments and clinical tests. Take anemia test as an example, drop the blood sample into copper sulfate solution. If the blood sample contains enough hemoglobin, the blood sample will sink rapidly; if the hemoglobin content is not enough, the blood sample will be suspended in the solution.

What is the difference between anhydrous copper sulfate and pentahydrate copper sulfate?
1.Their colors are different
Anhydrous CuSO4.5H2O is a white or gray-white powder, and CuSO4.5H2O is blue.
- They have the different chemical formula
The chemical formula of anhydrous copper sulfate is CuSO4, and that of pentahydrate copper sulfate is CuSO4·5H2O.
- Their structural stability are different
From the point of view of structural stability, CuSO4.5H2O is much better than anhydrous copper sulfate.

What is the reaction of humans after poisoning by eating anhydrous copper sulfate?
It can stimulate the impulse of the human’s nerve to transmit to the vomiting center of the medulla oblongata through a vagal and sympathetic nerve. Repeated and severe vomiting can lead to dehydration, shock, damage to the gastric mucosa, and even acute gastric perforation. Copper sulfate solution has strong local corrosion, can make the oral cavity, esophagus, gastrointestinal mucosa congestion, edema, ulcer, and erosion. Copper is also a neuromuscular poison. When copper enters the human body, it can cause systemic poisoning symptoms, damage the liver and kidney, cause steatosis and necrosis, and turn to inhibition after exciting the central nervous system.
The main poisoning manifestations are: nausea, vomiting, salivation, headache, dizziness, the special metal smell in the mouth, tongue coating, teeth, and gums can be dyed blue, abdominal pain, diarrhea, vomit, and excreta are also blue, jaundice, bloody urine, tachycardia, arrhythmia, pale complexion, liver pain, blood cell reduction, blood pressure drop, collapse, coma and dyspnea And so on. Prolonged poisoning time can cause liver damage, hematuria, oliguria, or anuria. In severe cases, there may be vascular paralysis, a sharp drop of blood pressure, rapid pulse, mania, delirium or coma, convulsion, and finally death of circulatory failure.
Why is copper sulfate the best choice for swimming pool disinfection?
It (CuSO4) is an effective and inexpensive algaecide with the advantages of high efficiency, low cost, and long duration.
The principle is that the toxicity of copper ions interferes with the photosynthetic reaction system of algae, making it unable to exercise photosynthesis normally and killing the spores of algae, so as to gradually achieve the goal of algae killing. Algae also need copper as a trace element, which must be excessive.
At the same time, it has another function, which is to make the swimming pool water as beautiful as seawater blue and make swimmers feel relaxed and happy.

Which kind of crystal is beautiful and easy to make?
CuSO4.5H2O and here is a common and simple method. I hope it works for you.
A half beaker saturated CuSO4.5H2O solution was prepared at room temperature. One end of a thin wire was tied to a small particle of copper sulfate crystal, and the other end was tied to the middle of a branch. Stick the branch on the beaker to make the small particles of CuSO4.5H2O suspended in the middle and lower part of the saturated CuSO4.5H2O solution. Then put the beaker in the refrigerator’s fresh-keeping room, and in a few days, you will get a half-finger size, pure copper sulfate pentahydrate crystal.
Can CuSO4.5H2O be added to skincare products?
It is generally used for skin conditioning in skincare products because a small amount of active copper ion can play a certain anti-inflammatory and bactericidal function, and as long as it meets the specified content standard, copper sulfate will not be dangerous to the human body.

Why not use the oxidation roasting method to produce copper sulfate?
The principle of this technology is that the ore is sulfated or oxidized and roasted at high temperatures to convert copper sulfide into a copper oxide which is easily soluble in dilute acid, and then it is prepared by acid leaching or ammonia leaching. In the process of roasting, a considerable amount of fuel is consumed, which will greatly increase the production cost and cause serious pollution to the environment.
What are the advantages and disadvantages of producing copper sulfate from sponge copper?
The method of producing CuSO4.5H2O with sponge copper has considerable economic benefits, less environmental pollution, and less cost. But it takes a long time to go through five processes, such as sulfuric acid dissolution, and has high requirements for raw materials and reagents.

What is the new technology for copper sulfate production?
Production of CuSO4.5H2O from copper concentrate. With this process, the yield of it can reach more than 95%. It has the characteristics of short process, less investment, simple operation, high copper utilization rate, and easy industrial implementation.
What is the principle of making copper sulfate by ammonia leaching method?
The ammonia leaching method is to dissolve copper and ammonia in oxidized copper ore to form stable complex ions, so as to separate them from impurities such as iron, other metal oxides, or alkaline earth metal carbonates. In this process, the amount of carbonated ammonia water is large, the price of carbonated ammonia water is cheap, and it has good selectivity for copper. Iron, aluminum, calcium, silicon, and other impurities can be removed and recycled. However, due to the strong smell of ammonia water. Therefore, the system must be recycled in a closed environment, with high requirements for equipment and large investment, which is not suitable for large-scale production.
Why use copper sulfate to make plant specimens?
The core of plant chlorophyll is a ring structure containing a magnesium ion. However, the chemical properties of magnesium ions are active and easy to present a free state. After the chemical reaction, the chlorophyll will be degraded, and the leaves will wither and yellow naturally. However, by contrast, copper ion is stable, once replaced by a magnesium ion, it will not easily occur chemical reaction. Chlorophyll containing copper ions will also show green. Therefore, It is often used in the color protection of plant specimens. The green processing of plants is a green processing technology, in which free copper ions in industrial raw materials such as copper sulfate and copper chloride form chlorophyll metal complexes with chlorophyll.

What are the applications of copper sulfate in aquaculture?
The breeders used copper sulphate as a feed additive to supplement copper for fish. Infeed production, thus on provide comprehensive nutrition for fish, it’s a necessity to supplement some metal parts, and copper is in addition needed among the tactic of fish growth and development. Therefore, sulphate is utilized as a result of the availability of copper in fish feed. it’s in addition used as medicine to kill some parasites. The toxicity of sulphate can kill some ciliates parasitic on fish, and it’s generally combined with metal among the tactic of breeding.
Copper sulfate can also be accustomed facilitate scale growth, that’s a smaller quantity utilised within the methodology of breeding.Generally, copper sulphate is employed before fish emerge, which might build the scales firmer and brighter.
What kind of chemical experiment is suitable for home?
As a company selling a variety of chemicals, We recommend the CuSO4.5H2O experiment to you. The process of making copper sulfate crystal is simple, and compared with other chemical experiments, the risk of CuSO4.5H2O experiments is very low. For this experiment, you only need to equip with some basic experimental materials. When you finished the experiment, the copper sulfate crystal has excellent ornamental value. It also can be used as a gift to relatives and friends.
In addition, we also need to remind you that the copper sulfate pentahydrate powder you purchased is toxic. If you accidentally put the powder, crystal, and solution into your mouth during the experiment, you should drink milk in time to relieve it. If you have any discomfort, please see a doctor in time. The surrounding environment should be well ventilated during the experiment and keep all kinds of equipment in good condition during the experiment.
Can I add a little copper sulfate to the food?
Yes, According to the relevant provisions of food safety laws in most countries, it is listed as a food processing aid to replace lead oxide in food processing, which helps to reduce the lead content in food. Therefore, enterprises can use food additive CuSO4.5H2O, but they must use food-grade CuSO4.5H2O instead of industrial copper sulfate. The use of industrial copper sulfate in food production will violate the laws and regulations of most countries, which is illegal.
At present, it is considered that the purity of industrial it is not enough, and it may contain lead, arsenic, cadmium, and other harmful elements. If it is used to process preserved eggs, it will have potential harm to the human body.

Is it feasible to produce copper sulfate from copper-containing waste?
Of course, it’s a feasible way. After copper leaching from the copper-bearing waste residue, waste printed circuit board and etching waste liquid, impurities such as lead, cadmium, nickel, and zinc enter into the solution together. The impurities can be selectively removed by solvent extraction method to realize multi-stage separation
This kind of valuable metal can be separated and recovered and can be operated continuously.
What is the hot spot of the copper sulfate production process in recent years?
As a professional supplier of CuSO4.5H2O, our main focus is bioleaching in recent years. Bioleaching is a new technology for the direct preparation of CuSO4.5H2O by selective bioleaching of copper from ore. The useful components of resources are oxidized or reduced by using their own oxidation and reduction characteristics in life activities. The separation of the useful components from the raw materials in the form of ion or precipitation in an aqueous solution is cleaner than the accumulation leaching by cyanide.
Because of its advantages of short process, simple equipment, and friendly environment, it has become a research hotspot in recent years.